When Is A Calorie Not a Calorie? Thinking Beyond Energy Balance

Despite a clear definition of what a calorie is, several commentators advocate the notion that a calorie is not always a calorie or that not all calories are equal. Statements like this grab people’s attention, as it suggests that the calories we eat may not all count in the same way, and our ignorance of this has meant we have been going about things all wrong. It also implies some magical properties associated with some calories compared to others. But what is the literal truth in these statements?
Firstly, calories are an established, quantifiable and clearly defined unit of energy, like any other designated unit, whether that be volts, kg, metres, or minutes. They do not change. A meal of 800kcal will still be 800kcal, irrespective of where those 800kcal are coming from (i.e. carbohydrate, fat, protein, fibre and alcohol). Not all dietary components generate the same diet-induced thermogenesis and net metabolisable energy. But, after factoring in these variations in diet-induced thermogenesis, the consumed 800kcal is all accountable in terms of energy balance. Hence, we do not just magically lose or gain calories.
Sadly, the truth is complicated and often oversimplified or misunderstood by many. Nevertheless, questioning whether a calorie is a calorie is helpful as it implies that the fate of the consumed calories is different depending on where these calories come from, which is technically accurate.
To better understand and contextualise what this means, we must look beyond just “calories in and calories out” and even beyond energy homeostasis, and fully appreciate energy metabolism which is crucial if wanting to predict the likely metabolic fate of the fat, protein, carbohydrate, and alcohol consumed. Indeed, such knowledge and understanding are fundamental to all of nutrition science and its application (health, disease, weight management, exercise science and beyond).
Beyond Energy Balance
We do not get our energy (calories) by plugging ourselves into the mains, but by burning the “fuel” that we have assimilated from food and drink. These fuels are our macronutrients (carbohydrate, fat, protein) and alcohol. Indeed, we know that our calculated energy intake hinges on knowing how much (e.g. in grams per day) of our “fuels” we are consuming and converting this into energy (calorie) equivalents. But rather than doing this, we can just consider the absolute amounts of carbohydrate, fat, protein and alcohol consumed, as this reflects our fuel (substrate) intake or, from a metabolic perspective, our intake of energy substrates.
On the other side of the energy balance equation, we consider how much energy we are expending like taking meter readings at home to calculate our energy bill. But instead, we can alternatively look at what fuels we are actually “burning” to provide this energy i.e. how much of our energy substrates carbohydrate, fat, protein and alcohol, have we burnt.

Combined, this fuel’s view of energy balance overall compares “fuels consumed versus fuels oxidised (burnt)”. Furthermore, you can view this on a fuel-by-fuel basis i.e. carbohydrate intake versus carbohydrate oxidation, fat intake versus fat oxidation, protein intake versus protein oxidation, and alcohol intake versus alcohol oxidation. Helpfully, we can measure substrate oxidation, as well as substrate intake.
Indirect calorimetry can assess oxygen consumption and CO2 production, and from this, we can calculate the respiratory exchange ratio (RER), which simply divides CO2 produced by O2 consumed. This ratio is also commonly termed your respiratory quotient (RQ), but this assumes that what you are measuring through breath reflects what is going in your tissues. Significantly, your measured RER (or RQ) will differ depending on what fuel you are oxidising.
We typically oxidise a mixture of fuels simultaneously, but the RER (or RQ) value indicates what fuel you are predominantly oxidising. Amino acids are typically not directly used as an energy substrate, so we often assume a fixed or constant protein contribution to the fuel mix or look at the non-protein RQ. Unless we are burning alcohol, the variability in the fuel mix is between fat and carbohydrate. An RQ (or RER) closer to 1.0 indicates carbohydrate oxidation predominantly, whereas closer to 0.7 implies more fat oxidation.
More usefully, we can use this tool to determine how our fuel mix changes, for example, after feeding or exercise. For example, feeding carbohydrate will typically shift you towards more glucose oxidation. With the help of metabolic chambers, we can also assess how 24-hour substrate oxidation rates may match up to the fuels consumed.
The Problem With Fat Balance
Let us now examine fuel balance over a typical 24-hour period for a typical healthy adult. We shall compare intake versus oxidation (or expenditure) for each substrate (carbohydrate, alcohol, protein and fat), and we shall compare intake versus oxidation (or expenditure), as depicted in the chart below.
So, when considering alcohol, metabolically, all alcohol (ethanol) consumed will be oxidised within 24 hours; we do not have ethanol stores in the body. Hence there is always a perfect balance between alcohol intake and alcohol oxidation. When we consider protein, we also know that, unless someone is undertaking growth or has infection or trauma, amino acid intake is near perfectly matched with amino acid oxidation. Indeed, this is the whole premise behind protein (nitrogen) balance, where nitrogen intake (mainly from protein) equals nitrogen excretion (urea and ammonium ions in the urine).
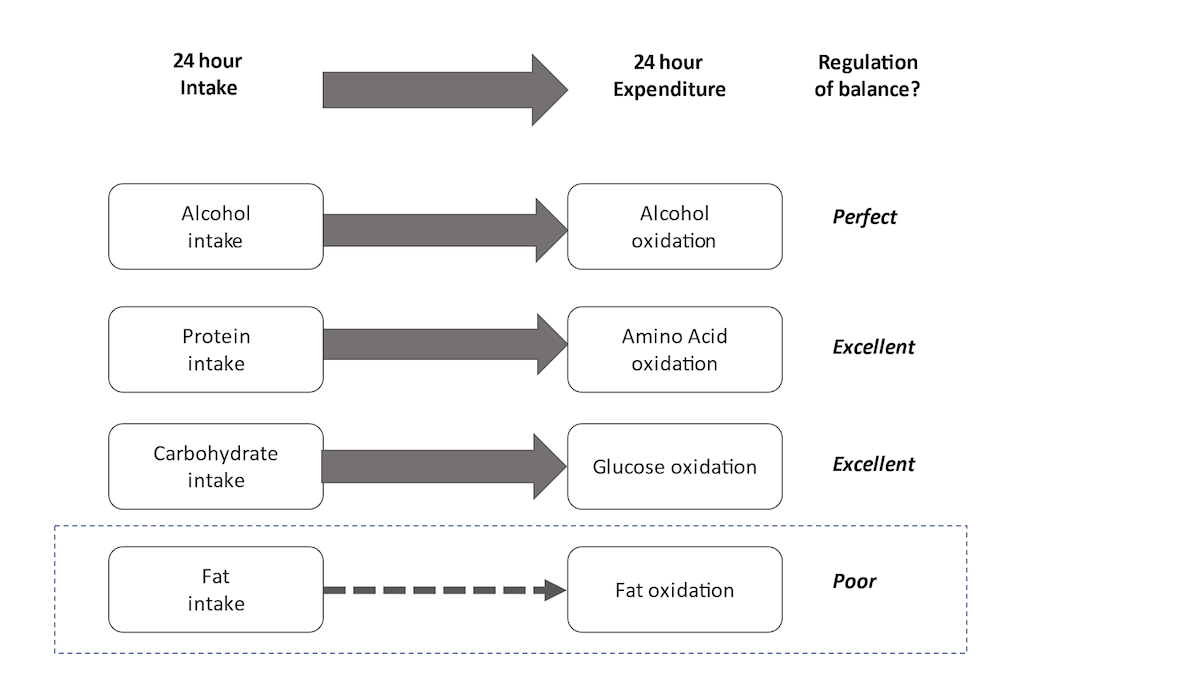
Carbohydrate intake will also lead to increased carbohydrate utilisation, predominantly through the action of insulin to maintain glucose homoeostasis. This regulation means, over a typical 24-hour period, carbohydrate intake is equal to carbohydrate oxidation. Overall utilisation and oxidation of alcohol, protein and carbohydrate are regulated to match their availability/supply. That is not the case for fat though.
Fat oxidation is influenced and controlled by the availability of other substrates, mainly carbohydrate and alcohol, not fat intake itself. Unlike the other energy substrates, there is no regulated balance between fat intake and fat oxidation. Fat oxidation will mainly occur in the absence of carbohydrate. Similarly, surplus carbohydrate may lead to net storage of fat and possibly increased fat synthesis (i.e. more available fat as a fuel).
Viewing energy balance in terms of substrate balance then helps us understand more about the operational consequences of any energy imbalance. It suggests any overall energy (calorie) imbalance will likely manifest as a change in fat balance, explaining why, certainly in the medium to long term, shifts in energy balance result in significant changes in body fat.
This is an edited excerpt from Dr. Adam Collins’ upcoming book the Metabolic Manual. To read more articles by Dr Adam click here.


















